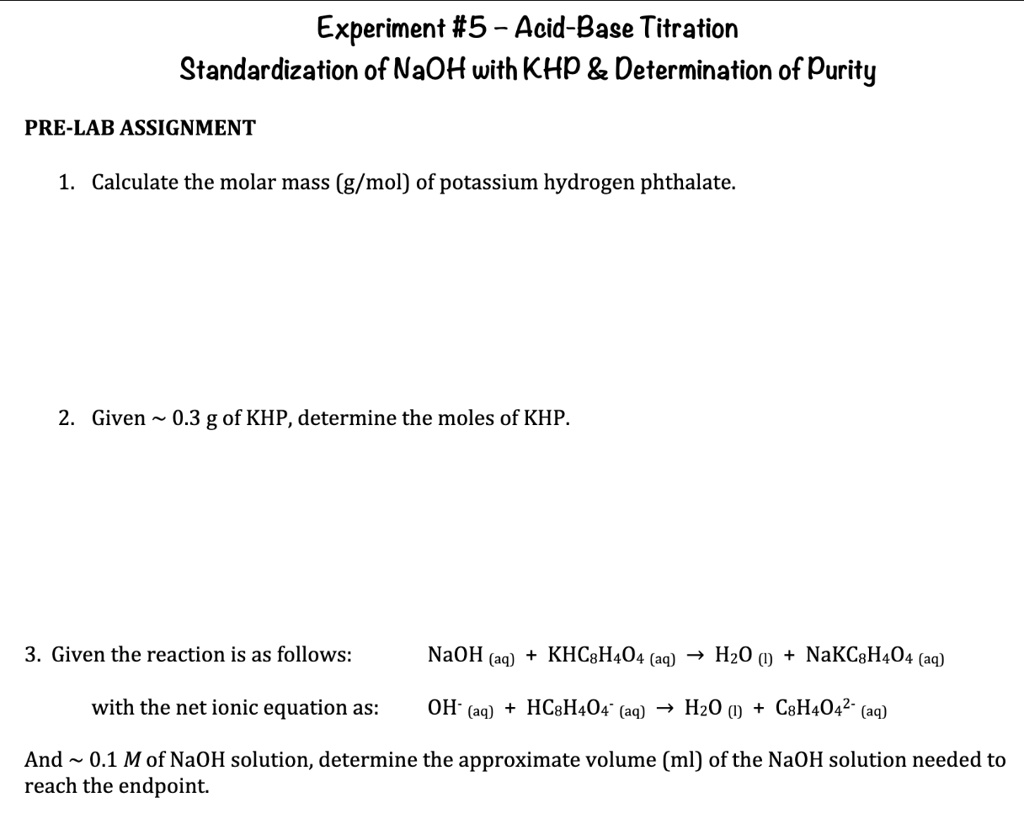
Titration Of Naoh With Khp . the titration of naoh with khp involves adding naoh from the burette to a known mass of khp in solution. use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via. The data from the titration is then used to calculate the molarity of the naoh. standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. Using the naoh you standardized, you will determine how much khp is in an impure. If the concentration of the khp solution is known accurately and the titration of a naoh solution with.
from www.numerade.com
standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via. standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. The data from the titration is then used to calculate the molarity of the naoh. the titration of naoh with khp involves adding naoh from the burette to a known mass of khp in solution. If the concentration of the khp solution is known accurately and the titration of a naoh solution with. Using the naoh you standardized, you will determine how much khp is in an impure.
SOLVED Question 3 Please Experiment 5 AcidBase Titration
Titration Of Naoh With Khp The data from the titration is then used to calculate the molarity of the naoh. The data from the titration is then used to calculate the molarity of the naoh. If the concentration of the khp solution is known accurately and the titration of a naoh solution with. the titration of naoh with khp involves adding naoh from the burette to a known mass of khp in solution. standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via. Using the naoh you standardized, you will determine how much khp is in an impure.
From www.numerade.com
SOLVED Question 3 What is the KHP NaOH mole ratio for the reaction Titration Of Naoh With Khp the titration of naoh with khp involves adding naoh from the burette to a known mass of khp in solution. Using the naoh you standardized, you will determine how much khp is in an impure. standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. standardization of a naoh solution. Titration Of Naoh With Khp.
From asideload7.gitlab.io
Nice Khp Naoh Titration Calculations List Of All Physics Formulas Titration Of Naoh With Khp standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. Using the naoh you standardized, you will determine how much khp is in an impure. If the concentration of the khp solution is known accurately and the titration of a naoh solution with. use the virtual laboratory to standardize an unknown. Titration Of Naoh With Khp.
From www.visionlearning.com
Acids and Bases I Math in Science Visionlearning Titration Of Naoh With Khp use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via. standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. The data from the titration is then used to calculate the molarity of the naoh. standardize a sodium hydroxide (naoh) solution using titration. Titration Of Naoh With Khp.
From www.chegg.com
Solved TITRATION ⋅ STANDARDIZATION OF SODIUM HYDROXIDE Titration Of Naoh With Khp standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. The data from the titration is then used to calculate the molarity of the naoh. standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. Using the naoh you standardized, you will determine how. Titration Of Naoh With Khp.
From www.numerade.com
SOLVED Part 1 KHP Titrations Question 2 (1 point) A student Titration Of Naoh With Khp If the concentration of the khp solution is known accurately and the titration of a naoh solution with. the titration of naoh with khp involves adding naoh from the burette to a known mass of khp in solution. standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. use the. Titration Of Naoh With Khp.
From www.numerade.com
SOLVED A student carried out two titrations using a NaOH solution of Titration Of Naoh With Khp Using the naoh you standardized, you will determine how much khp is in an impure. If the concentration of the khp solution is known accurately and the titration of a naoh solution with. standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. The data from the titration is then used to. Titration Of Naoh With Khp.
From www.vrogue.co
The Standardization Of Naoh And Khp Assay vrogue.co Titration Of Naoh With Khp The data from the titration is then used to calculate the molarity of the naoh. the titration of naoh with khp involves adding naoh from the burette to a known mass of khp in solution. If the concentration of the khp solution is known accurately and the titration of a naoh solution with. standardization of a naoh solution. Titration Of Naoh With Khp.
From www.studocu.com
PostLab Questions of Titration of KHP with NaOH solution Post Lab Titration Of Naoh With Khp use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via. standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. If the concentration of the khp solution is known accurately and the titration of a naoh solution with. Using the naoh you standardized, you. Titration Of Naoh With Khp.
From www.numerade.com
SOLVED TITRATION ACIDBASE DATA Preparation Sodium Hydroxide Solution Titration Of Naoh With Khp standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. If the concentration of the khp solution is known accurately and the titration of a naoh solution with. The data from the titration is then used to calculate the molarity of the naoh. standardize a sodium hydroxide (naoh) solution using titration. Titration Of Naoh With Khp.
From www.vrogue.co
Nice Khp Naoh Titration Calculations List Of All Phys vrogue.co Titration Of Naoh With Khp standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. If the concentration of the khp solution is known accurately and the titration of a naoh solution with. The data from the titration is then used to calculate the molarity of the naoh. the titration of naoh with khp involves adding. Titration Of Naoh With Khp.
From www.chegg.com
Solved Data Table I Standardization of a NaOH Solution (0.5 Titration Of Naoh With Khp The data from the titration is then used to calculate the molarity of the naoh. Using the naoh you standardized, you will determine how much khp is in an impure. standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. standardization of a naoh solution with potassium hydrogen phthalate (khp) and. Titration Of Naoh With Khp.
From www.numerade.com
SOLVED Estimate the mass of KHP (KHC8H4O4) that will require 20 mL of Titration Of Naoh With Khp Using the naoh you standardized, you will determine how much khp is in an impure. If the concentration of the khp solution is known accurately and the titration of a naoh solution with. standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. the titration of naoh with khp involves adding. Titration Of Naoh With Khp.
From www.vrogue.co
Standardization Of Naoh Acid Base Titration Objective vrogue.co Titration Of Naoh With Khp Using the naoh you standardized, you will determine how much khp is in an impure. use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via. standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. the titration of naoh with khp involves adding. Titration Of Naoh With Khp.
From asideload7.gitlab.io
Nice Khp Naoh Titration Calculations List Of All Physics Formulas Titration Of Naoh With Khp use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via. Using the naoh you standardized, you will determine how much khp is in an impure. the titration of naoh with khp involves adding naoh from the burette to a known mass of khp in solution. If the concentration of the khp. Titration Of Naoh With Khp.
From shotprofessional22.gitlab.io
Ace Khp And Naoh Balanced Equation Physics Wallah 12th Class Chemistry Pdf Titration Of Naoh With Khp The data from the titration is then used to calculate the molarity of the naoh. If the concentration of the khp solution is known accurately and the titration of a naoh solution with. Using the naoh you standardized, you will determine how much khp is in an impure. standardization of a naoh solution with potassium hydrogen phthalate (khp) and. Titration Of Naoh With Khp.
From www.vrogue.co
Standardization Of Naoh With A Khp Solution Acid Base vrogue.co Titration Of Naoh With Khp standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. The data from the titration is then used to calculate the molarity of the naoh. the titration of naoh with khp involves adding. Titration Of Naoh With Khp.
From plot.ly
KHP and NaOH Titration Curve line chart made by Kylclk plotly Titration Of Naoh With Khp the titration of naoh with khp involves adding naoh from the burette to a known mass of khp in solution. use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via. standardization of a naoh solution with potassium hydrogen phthalate (khp) and titration of vinegar with standardized naoh. The data from. Titration Of Naoh With Khp.
From solvedlib.com
Preparing standard KHP and titration with NaOHMolar … SolvedLib Titration Of Naoh With Khp The data from the titration is then used to calculate the molarity of the naoh. use the virtual laboratory to standardize an unknown naoh solution (approximately 0.2m) to four significant figures via. standardize a sodium hydroxide (naoh) solution using titration of potassium hydrogen phthalate (khp) using a calibrated buret. standardization of a naoh solution with potassium hydrogen. Titration Of Naoh With Khp.